Every meal prepared, every packaged snack, and every bottled beverage begins with a critical foundation: Good Manufacturing Practice (GMP). GMP for food industry, is not an optional standard—it is the essential framework that ensures products are safe, consistent, and produced under hygienic conditions from raw material to final package.
More than just rules, Good Manufacturing Practice or GMP for Food Industry comprises the fundamental prerequisite programs that prevent contamination and adulteration. It is the bedrock upon which advanced food safety systems like HACCP are built. Implementing GMP means proactively designing quality and safety into every step of your process, rather than just testing for failures after the fact.
For food factory managers, QA professionals, and business owners in Malaysia and beyond, navigating GMP requirements is key to operational integrity and market access. Compliance is crucial for meeting local food safety standards, exporting to regulated markets, and, most importantly, protecting consumers and your brand from the catastrophic risks of a food safety incident.
This complete guide is your definitive resource. We will explain the core principles of GMP specific to food manufacturing, detail its non-negotiable link with HACCP, and provide a clear step-by-step implementation roadmap. Finally, you will understand how to build lasting competency through targeted GMP training for your team. Let’s begin building your foundation of food safety.
What is Good Manufacturing Practice (GMP) in the Food Industry?
Good Manufacturing Practice, GMP for food industry is a systematic, operational framework designed to ensure that food products are consistently produced, processed, and controlled according to stringent quality and safety standards. Think of GMP as the essential “rules of hygiene and discipline” for every food factory—it governs everything from the cleanliness of walls and equipment, to the hygiene of personnel, to the way raw materials are stored and handled.
Unlike a focus solely on testing finished products, GMP is preventive by design. It establishes the fundamental environmental and operational conditions—known as Prerequisite Programs (PRPs)—that must be in place to prevent contamination, mix-ups, and errors before they can affect food safety. In essence, GMP builds the foundational hygiene barrier that protects your production line from biological, chemical, and physical hazards.
For any food business—from a small-scale local processor to a large export-oriented manufacturer—implementing GMP is the critical first step in demonstrating a commitment to safe food. It is a compliment food safety system to Malaysia Halal Certification scheme, and it forms the mandatory foundation upon which more advanced, risk-based systems like HACCP (Hazard Analysis and Critical Control Points) are built. Without solid GMP, your HACCP plan is built on shaky ground.
Why GMP is the Non-Negotiable Foundation of Every Food Safety Plan
You cannot build a sturdy house on sand. Similarly, you cannot build an effective, reliable food safety management system without the solid foundation of GMP. This principle is recognized globally by standards like ISO 22000 and FSSC 22000, and it is a core directive of the Codex Alimentarius, the international food safety benchmark.
Here’s why GMP is non-negotiable:
It Controls the “Basic Hygiene” Environment:
HACCP is designed to address specific, significant hazards at critical control points. However, HACCP cannot and should not manage general hygiene issues like dirty floors, poor personnel habits, or inadequate pest control. These are the domain of GMP. GMP controls the overall environment so that your HACCP plan can focus on the critical process-specific risks.
It is a Regulatory and Market Access Requirement:
In Malaysia, the Ministry of Health (MOH) and the Department of Islamic Development Malaysia (JAKIM) for Halal certification require robust GMP as part of their assessments. For export, markets like Singapore, the European Union, and the United States mandate GMP compliance (e.g., under FDA’s 21 CFR Part 110) as a basic entry ticket. No GMP, no market access.
It Directly Prevents the Most Common Causes of Recalls:
The majority of food recalls and safety incidents stem from failures in basic hygiene and operational control—precisely what GMP governs. This includes allergen cross-contact due to poor sanitation, microbial contamination from unclean equipment, and foreign material from faulty maintenance. A strong GMP system is your primary defense against these costly, reputation-destroying events.
It Creates a Culture of Daily Discipline:
GMP translates food safety from a theoretical concept into daily, tangible actions. It defines the standard for every employee, from the factory floor to the quality lab. This ingrained culture of discipline and attention to detail is what enables more complex systems to function reliably.
The 10 Core Principles GMP for Food Industry
Good Manufacturing Practice is built on universal principles that apply to every food facility, regardless of size or product. These ten principles form the pillars of your food safety system. Mastery of each is non-negotiable for compliance, audit success, and, most importantly, producing safe food.
Principle 1: Suitable Premises and Location
Your facility’s design is your first line of defense. Buildings must be located in environments that minimize contamination risks (e.g., away from industrial pollutants, flooding zones). The layout must facilitate a logical, one-way flow of materials and personnel—from raw intake to finished goods—to prevent cross-contamination. Floors, walls, and ceilings must be constructed from impervious, cleanable materials with proper drainage.
Principle 2: Fit-for-Purpose Equipment
All equipment contacting food must be made of food-grade, corrosion-resistant materials (like stainless steel) and be designed for easy cleaning, maintenance, and inspection. Critical instruments (e.g., thermometers, pH meters) must be regularly calibrated. A preventive maintenance schedule is mandatory to avoid breakdowns that could compromise safety.
Principle 3: Clearly Defined Cleaning and Sanitation (C&S)
A documented, validated Cleaning and Sanitation program is the heartbeat of GMP. It must specify: what is to be cleaned, how, with which chemicals (at correct concentrations), when (frequency), and by whom. Procedures must include methods to verify cleaning effectiveness (e.g., ATP swab testing) and prevent chemical contamination of food.
Principle 4: Rigorous Pest Control
An Integrated Pest Management (IPM) program is essential. This proactive strategy focuses on prevention by denying pests food, water, and shelter through facility integrity (sealed cracks, air curtains). It is supported by monitoring (trap logs) and controlled interventions. Pesticides must be used as a last resort and never risk contaminating product.
Principle 5: Controlled Water, Air, and Steam Quality
Water used as an ingredient, for cleaning, or for steam generation must be of potable quality and regularly tested. Compressed air that contacts food or packaging must be filtered and free of oil and moisture. Steam used in direct contact with food must be culinary-grade. These utilities are silent vectors of contamination if uncontrolled.
Principle 6: Comprehensive Personnel Hygiene and Health
People are the greatest potential source of contamination. Strict hygiene policies must govern: protective clothing (hairnets, gloves, dedicated footwear), handwashing procedures, jewellery prohibition, and illness reporting. Regular training is critical to instill a culture where every employee understands their direct role in food safety.
Principle 7: Strict Control of Raw Materials and Suppliers
You cannot produce safe food from unsafe ingredients. A formal supplier approval and monitoring program is required. All incoming materials must be inspected, tested (as per specification), and stored correctly. A “First-In, First-Out” (FIFO) system must be enforced. Traceability—the ability to track any ingredient from receipt to finished product batch—is paramount.
Principle 8: Validated Manufacturing Processes and Controls
Every step of your process must be controlled within defined, science-based limits. This includes critical parameters like time, temperature, pH, and pressure. Processes must be validated to prove they consistently achieve the intended safety and quality result. Any deviation requires investigation and documented corrective action.
Principle 9: Systematic Storage, Distribution, and Transportation
Finished products must be stored under specified conditions (temperature, humidity) to prevent deterioration. Warehouses must be orderly, clean, and secure. Distribution vehicles must be suitable for food transport, clean, and capable of maintaining required temperatures (e.g., chilled or frozen chains). Records of storage and transport conditions must be maintained.
Principle 10: Meticulous Documentation and Record Keeping
“If it isn’t written down, it didn’t happen.” This is the golden rule of GMP for Food Industry. Every procedure, instruction, and specification must be documented. Every action (cleaning, calibration, production, training) must be recorded. These records provide evidence of control, enable traceability, and are the primary source of truth during an audit. Records must be legible, accurate, and retained for a defined period.
Implementing the Principles: From Theory to Practice
Understanding these principles is the first step; implementing them consistently is where excellence is achieved. This requires:
Risk Assessment: For each principle, conduct a risk assessment of your current operations. Where are the gaps?
Documented Procedures: Develop clear Standard Operating Procedures (SOPs) for each area (e.g., “SOP for Handwashing,” “SOP for Line Clearance and Sanitation”).
Training and Competency: Every employee must be trained on the SOPs relevant to their role. Training effectiveness must be verified.
Monitoring and Verification: Use checklists, logs, and audits to ensure procedures are followed daily.
Management Review: Senior management must regularly review GMP performance data and commit resources to continuous improvement.
Understanding the principles is the knowledge; implementing them is the action. This section provides your actionable, step-by-step roadmap to transform your food facility’s operations from ad-hoc practices to a systematic, GMP-compliant food safety culture. We will also clarify the critical, non-negotiable link between GMP and HACCP.
How to Implement a GMP for Food Factory: A 5-Step Guide
Implementation is a project that requires management leadership, resources, and a methodical approach. Follow this five-phase roadmap.
Phase 1: Management Commitment & Baseline Gap Analysis
Action: Secure a formal, documented commitment from top management. This includes defining a clear Food Safety Policy and allocating budget and authority.
Execution: Conduct a comprehensive Gap Analysis. Audit your current state against the 10 GMP principles and relevant standards (MS1514, Codex). This report becomes your project blueprint, identifying every “what we have” vs. “what we need” gap.
Phase 2: Develop the Documentation Backbone
Action: Create your Quality Management System (QMS) Documentation.
Execution:
Top-Level Manual: Draft a Food Safety & Quality Manual outlining your system’s scope and structure.
System Procedures: Write procedures for key management processes (e.g., “Document Control,” “Internal Audit,” “Corrective Action”).
SOPs & Work Instructions: Develop clear, visual Standard Operating Procedures (SOPs) and work instructions for every critical task defined in your gap analysis (cleaning, calibration, production line setup, etc.).
Phase 3: Facility & Infrastructure Modification
Action: Close the physical and environmental gaps identified in Phase 1.
Execution: This is often the most capital-intensive phase. Execute projects like: repairing flooring, installing adequate handwashing stations, segregating raw and finished goods areas, improving pest-proofing, and calibrating/repairing equipment. The goal is to create an environment that enables compliance.
Phase 4: Training, Rollout, and Operational Launch
Action: Translate documents into action through rigorous training and change management.
Execution:
Train All Personnel: Roll out mandatory GMP Awareness Training for every employee, followed by role-specific SOP training. This is where your team’s expertise is critical.
Dry Run & Refine: Run new procedures in a controlled manner before full launch. Gather feedback and refine SOPs.
Full Implementation: Go live with the new system on a defined date. Management must be visibly present to enforce the new standards.
Phase 5: Verification, Audit, and Continuous Improvement
Action: Move from implementation to maintenance and growth.
Execution:
Internal Audit: Schedule and conduct your first internal GMP audit within 3 months of launch. This checks for compliance and system effectiveness.
Management Review: Present audit findings, monitoring data, and non-conformances to top management. Decide on resource allocation for improvements.
Cyclical Refinement: Use the Plan-Do-Check-Act (PDCA) cycle. The system is never “finished”; it is continuously improved based on data
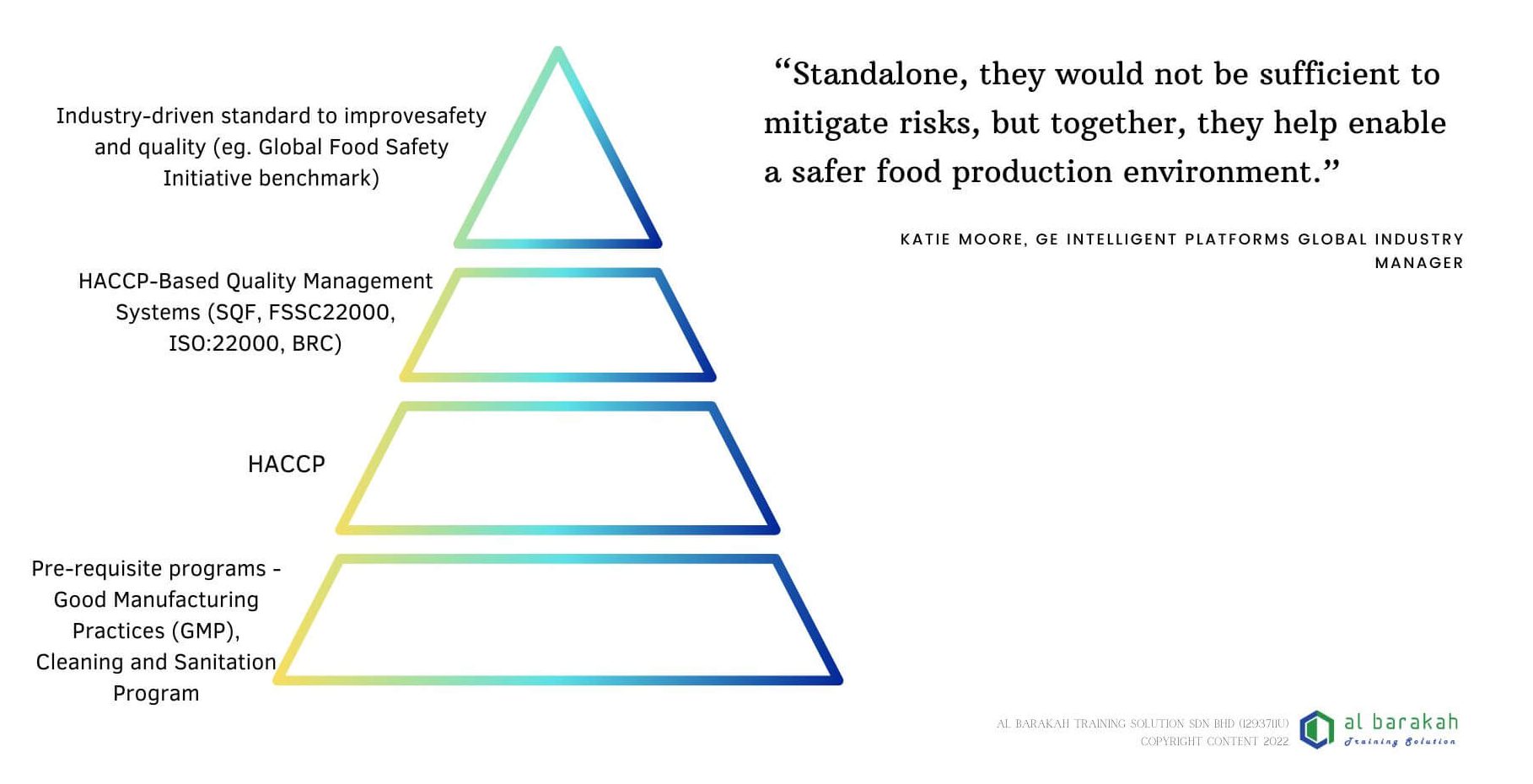
The Critical Link: Using Your GMP Foundation to Build a HACCP Plan
A HACCP plan without GMP is a castle built on sand. The relationship is hierarchical and interdependent.
The Hierarchy of Control:
Level 1: Good Manufacturing Practice (GMP): These are your Prerequisite Programs (PRPs). They control the general factory environment and create the baseline hygienic conditions. They manage widespread, low-level hazards (e.g., general pathogen contamination from dirty equipment).
Level 2: Operational Prerequisite Programs (oPRPs): These are control measures, often derived from GMP, that manage a specific significant hazard but are not based at a Critical Control Point (CCP). They are monitored but may not have critical limits (e.g., a validated allergen cleaning procedure).
Level 3: HACCP Plan (CCPs): This is your targeted, risk-based system for the most significant hazards that require the highest level of assured control at specific points in the process (e.g., pasteurization temperature/time to eliminate Listeria).
The Sequential Process:
First, establish and verify your GMP/PRPs. You cannot conduct an accurate hazard analysis in a chaotic, uncontrolled environment.
Then, assemble your HACCP team and follow the 12 steps, starting with a product description and process flow diagram.
During your Hazard Analysis (Principle 1), you will identify which hazards are controlled by your existing, effective GMP programs. These hazards are prevented at the PRP level and do not need a CCP.
The remaining, unprevented significant hazards are what your CCPs will control.
In practice: A robust GMP system for food industry addressing Principle 3 (Cleaning) may prevent general microbial contamination. Your HACCP plan can then focus its CCP on the precise thermal step that guarantees the elimination of the specific pathogen of concern for that product. Your GMP handles the background “noise”; your HACCP targets the specific “signal.”
Key Takeaway: Attempting HACCP without solid GMP will result in an overburdened, ineffective HACCP plan with too many CCPs trying to do the job of basic hygiene. A strong GMP foundation allows your HACCP plan to be focused, efficient, and scientifically sound.
“A robust GMP system as defined by MS 1514:2022 is the essential foundation for a compliant HACCP system under MS 1480. It’s important to note that the HACCP standard itself is updated periodically to reflect best practices. The latest revision, Malaysia’s MS 1480:2025, places even greater emphasis on prerequisite programs like GMP and introduces new requirements for hazard analysis. To understand how these updates might impact your integrated food safety management system, review our analysis here: Key Changes in MS 1480:2025.”
Preparing for a GMP Audit: Checklist for Audits
A GMP system is not a “set and forget” project. Its true strength is proven through verification, challenged by audits, and refined through a relentless commitment to getting better. This section provides the tools to validate your system, sail through audits, and embed a culture of continuous improvement.
An audit is not a punishment; it is a rigorous health check for your food safety system. Being “audit-ready” at all times is the mark of a mature operation.
The Three Types of GMP Audits:
Internal Audit (First-Party): Your own team audits against your own standards. This is a self-check for compliance and improvement opportunities. It should be conducted regularly by trained, objective internal auditors.
External Customer Audit (Second-Party): A buyer or potential client audits you to ensure you can meet their specific standards and contractual requirements. These are often detailed and product-specific.
Certification/Regulatory Audit (Third-Party): An certification body (e.g., Sirim, SGS) or a government regulator (e.g., MOH) audits you against a formal standard. Success leads to certification or license renewal.
The Universal Audit Preparedness Checklist:
Pre-Audit (Weeks/Months Before):
Documentation Review: Ensure all SOPs, manuals, and records are up-to-date, properly approved, and readily accessible. Check for revision control.
Internal Audit Closure: Verify all findings from your last internal audit have been closed with effective corrective actions.
Training Records: Confirm all personnel training is current, documented, and signatures are in place.
Facility Walk-Through: Conduct a GMP inspection using a fresh set of eyes. Look for structural issues, housekeeping failures, and any out-of-place items.
During the Audit (The Audit Day):
Assign a Knowledgeable Guide: Designate a lead responder (often the QA Manager) to accompany the auditor. This person knows where documents are and can provide clear explanations.
Be Honest and Transparent: If a non-conformance is found, acknowledge it. Explain your understanding and your proposed corrective action. Never hide information.
Show Evidence, Not Just Talk: When asked a question, lead the auditor to the relevant SOP and then to the records proving it is followed.
Interview Preparedness: Ensure staff are briefed and understand they may be asked about their daily duties. They should answer based on their training and the actual SOPs.
Post-Audit (After the Report):
Formal Response: For every finding (major or minor), submit a Corrective and Preventive Action (CAPA) plan. This must include: Root Cause Analysis, Immediate Correction, Action to Prevent Recurrence, and Verification of Effectiveness.
Systemic Review: Use audit findings as a powerful tool for management review. Ask: Do we need to update a procedure? Invest in new equipment? Provide re-training?
Corrective Actions and Maintaining Your GMP System
The goal is not just to fix problems, but to eliminate their root causes and prevent recurrence. This is the essence of Continuous Improvement.

The CAPA Process: A Step-by-Step Model
Identify the Non-Conformance: This could be from an audit, a customer complaint, a monitoring result (e.g., failed swab test), or an internal observation.
Immediate Correction (Containment): Take short-term action to isolate the problem (e.g., quarantine affected product, clean a spill).
Root Cause Analysis (RCA): This is the critical step. Use tools like the “5 Whys” to drill down past the symptom to the true system failure. Why was the procedure not followed? Was the training inadequate? Was the SOP unclear? Was there time pressure from production?
Plan and Implement Corrective & Preventive Actions:
Corrective Action: Address the specific root cause (e.g., re-train the specific employee on the SOP).
Preventive Action: Broaden the solution to prevent it anywhere else (e.g., update the SOP for clarity, add the topic to the annual refresher training for all staff).
Verify Effectiveness: After a defined period, check that the action worked. Re-audit the area, review relevant records, and confirm the non-conformance has not recurred.
Document Everything: The entire CAPA journey—from finding to verification—must be recorded. This is your evidence of a living, learning system.
Building a Culture of Continuous Improvement
Empower Employees: Create a simple system for frontline workers to report near-misses or suggestions without fear. They see the process daily.
Regular Management Reviews: Schedule quarterly reviews where data from audits, monitoring, complaints, and CAPAs are analyzed to spot trends and direct resources.
Celebrate Improvements: Recognize teams that identify and solve problems. This reinforces the desired behavior.
Essential GMP for Food Industry Training for Every Role
The most sophisticated GMP system on paper is worthless without competent people to execute it. GMP in food industry Training is not an expense; it is the critical investment that transforms your documentation into daily, safe practice. This final section outlines how to build a structured training program that turns your workforce into your strongest food safety asset.
Effective training is not one-size-fits-all. It must be role-specific, practical, and reinforced. The table below outlines the core GMP knowledge required at different levels of your organization:
| Role / Level | Primary GMP Focus | Key Training Objectives | Recommended Training Type |
|---|---|---|---|
| QA/QC & Technical Staff | System Management & Compliance | Master audit techniques, root cause analysis, documentation control, and regulatory updates. | GMP Verification & Internal Audit Training |
| Production Supervisors & Line Leaders | Daily Execution & Supervision | Deep understanding of SOPs, real-time problem identification, and coaching frontline staff. | GMP Implementation & Supervision Training |
| All Food Handlers & Operators | Foundational Hygiene & Procedure | Personal hygiene, basic SOP adherence (cleaning, line setup, reporting), and understanding “why” behind rules. | Mandatory GMP Awareness & Hygiene Training |
Advanced GMP & Internal Auditor Training: For QA Staff and System Guardians
To maintain and improve your system, you need in-house experts. This training moves beyond basic compliance to system mastery and verification skills.
Core Curriculum Must Cover:
Audit Principles & Techniques: How to plan, execute, and report an internal audit against GMP standards.
Root Cause Analysis Mastery: Applying tools like 5 Whys, Fishbone Diagrams, and Pareto Analysis to find true systemic causes.
Documentation & Record Review: Developing an eye for detecting inconsistencies and gaps in records.
Corrective Action Management: Learning how to write effective CAPAs that prevent recurrence.
Regulatory Interpretation: Staying updated on changes to MOH, Local regulations, MS1514, Codex, and other relevant standards.
Outcome: A competent internal audit team that can proactively find and fix weaknesses, preparing your facility seamlessly for external audits.
This level of expertise is developed through dedicated, advanced instruction.
[Build your internal audit capability with our expert-led GMP Implementation & Auditor Training.]
Implementing a Sustainable Training Program
Training is not a one-time event. A sustainable program requires:
Training Needs Analysis (TNA): Annually assess skills gaps based on audit findings, new equipment, or changed regulations.
Competency Verification: Use quizzes, practical demonstrations, and supervisor observations to confirm understanding—not just attendance.
Refresher Training: Schedule mandatory refreshers annually or bi-annually to combat complacency and reinforce key messages.
Record Keeping: Maintain meticulous training records for each employee—the cornerstone of proof during an audit.
Your Next Step Towards GMP Excellence In Food Industry
Building a compliant, resilient food safety culture is a journey that begins with knowledge and is cemented through competent execution. This guide has provided you with the blueprint—from principles and implementation to audit readiness and workforce development.
The most critical success factor is getting your team’s training right.
Let’s Assess Your Training Needs.
Every facility is unique. A training program must be tailored to your specific processes, risks, and team structure.
Schedule a Free 30-Minute GMP Food Industry Training Consultation with Our Food Safety Specialists.
We will help you:
Review your current training needs and identify gaps.
Map roles to the appropriate training modules.
Propose a phased training plan to build competency without disrupting production.
Protect your consumers, your brand, and your business. Invest in competence today.
Click Here to Inquire Now or email us directly at sa***@************om.my to discuss the best training option for your team and secure your dates.







